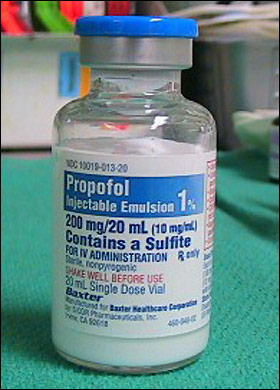
All provincial health authorities and private hospitals in Papua New Guinea have been warned of the use of Propofol Injection IP 10 Milligrams (mg) per Milliliter (mL) vial (1 per cent W/V).
This is according to a circular number 50 of 2021 that was issued on 17 August by Health Secretary, Dr Osborne Liko following the seven serious and fatal adverse incidents at the Port Moresby General Hospital Operation Theatre reported on 12 August caused by an anesthetic drug believed to be manufactured in India.
These cases included the death of four patients.
Last Friday, PMGH CEO Dr Paki Molumi confirmed that he is aware of this circular and responded last week by saying the death of the four patients after undergoing surgery in the middle of this month, August, was related to the administration of a batch of anesthetics.
Among them was a 14-year-old boy, whose death had come as a shock and disappointment to the families and the public. The boy died after undergoing an eye surgery at the hospital on 12 August.
Dr Molumi said investigation is still underway and PMGH will ensure that these investigations thorough and independent to assist and bring closure to these cases.
“The Health Department is taking this matter seriously and has requested for immediate identification and isolation of this batch of drugs from the entire procurement supply chain in both private and public hospitals,” Dr Liko said in the circular.
“All chief executive officers of PHAs, public, private and mission hospitals, all director medical services and clinical directors of public and private hospitals, all officers in charge (OIC), operating theatre managers of public and private operating theatres, all store technical advisers, pharmacy managers and OIC pharmacists in the hospitals pharmacies dispensaries, all pharmaceutical importers, wholesalers and distributors and manager country operations UNOPS and all other international health partners are urged to immediately identify and quarantine this batch from the entire procurement supply chain system both in public and private sectors in the country.”
As per the circular, the product name is Propofol Injection IP 10 mg/mL vial (1 per cent W/V). Manufacture is Cooper Pharma Limited, India. Batch/Lot Number LE21007, Manufacturing Date is 04/2021 and Expiry Date 03/2023.
Dr Liko said the Health Department through the Procurement and Regulatory team is conducting a preliminary and safety assessments to identify the cause of the serious and fatal adverse events during the use of the product.
“The Health Department is treating this as a very serious incident and full cooperation will be requested from all parties to fully establish the cause,” he said.
SOURCE: POST COURIER/PACNEWS